The guidance document was issued by the Center for Drug Evaluation and Research of the FDA and was intended for IRBs and clinical investigators.
The Food and Drug Administration (FDA) has recently issued an updated guidance report. This is a detailed document that provides instruction describing the potential to access an Institutional Review Board (IRB), a general term describing a group evaluating results of data collected during medical testing for SARS-CoV-19.
“During the COVID-19 public health emergency, the Agency has received a substantially increased volume of individual patient expanded access requests for COVID-19 investigational drugs. Although FDA has issued guidance on expanded access requests, including expanded access for individual patients, the Agency is aware that Institutional Review Boards (IRBs) seek clarity regarding the key factors and procedures IRBs should consider when reviewing individual patient expanded access submissions, including for reviews conducted by a single member of the IRB, to fulfill its obligations under 21 CFR Part 56. Therefore, FDA is issuing this guidance to provide recommendations regarding the key factors and procedures IRBs should consider when reviewing expanded access submissions for individual patient access to investigational drugs for treating COVID-19.”
The guidance document was issued by the Center for Drug Evaluation and Research of the FDA and was intended for IRBs and clinical investigators. An IRB reviews the procedures and steps taken during a medical review to ensure that the patient’s rights were considered and respected during the examination.
RELATED: FDA Steps Up In Ongoing Coronavirus Health Crisis
The purpose of IRB review is to assure, both in advance and by periodic review, that appropriate steps are taken to protect the rights of individuals as patients, when these patients are participating as subjects in the research.
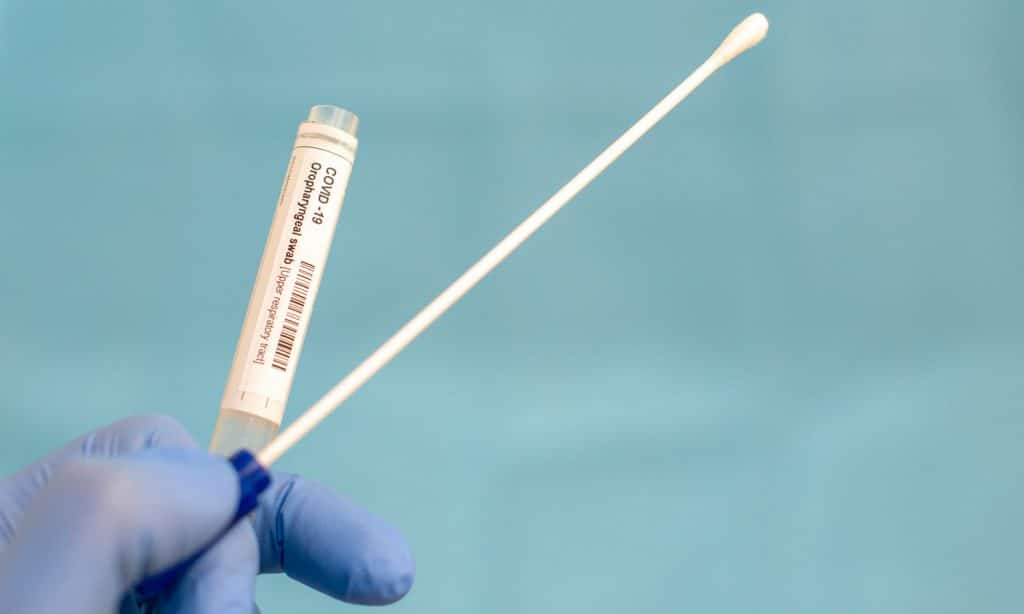
The guidance also applies to investigational drugs. Investigational drugs may be approved by the FDA for use in one disease or condition, but will still be considered “investigational” in treating other diseases or conditions (in other situations, this might be called an experimental or an investigational agent)
By the time the national emergency was declared on March 13, the requests by physicians to treat their COVID-19 patients with investigatory drugs had significantly increased. The ability to treat patients with investigatory drugs falls under the FDA’s umbrella regulations for the patient’s expanded access pathway. This is sometimes called “compassionate use guidance.” This allows a patient with a serious disease to gain access to an investigational drug or biological product.
The unprecedented spread — and strength and sheer volume — of COVID-19 cases in the U.S. are among the factors that necessitated this decision.