Warning Letters are a fairly minor enforcement action. However, the FDA also has the authority to levy fines and even refer cases to the Department of Justice.
The Food and Drug Administration (FDA) has consistently taken the position that cannabidiol derived from hemp (CBD) cannot be marketed or sold as a dietary supplement or food. The FDA’s position is based on the fact that CBD has been approved for use in a pharmaceutical drug and therefore cannot legally be sold in any ingestible form.
In addition, if a CBD company makes any health or wellness claims (e.g., CBD can help you relax, CBD cures back pain) then the FDA will consider the product an unauthorized drug.
The FDA has enforced this position against companies selling CBD by issuing Warning Letters, which, as the name suggests, warns companies regarding the improper sale of adulterated and misbranded foods. Despite the consistency from the FDA, there has been a recent uptick from the agency in sending out Warning Letters to CBD companies.
RELATED: The FDA Sets Targets On Delta-8
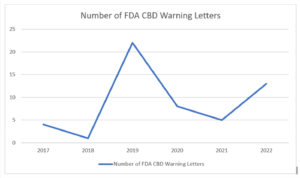
*note: one letter was sent in 2022 to a company selling delta-8 and is not reflected in this graph.
As you can see, the Warning Letters spiked in 2019, which coincides with the passage of the 2018 Farm Bill in December 2018. With the rise of Covid, the FDA was preoccupied with the pandemic and the amount of Warning Letters decreased significantly. However, it appears that the FDA is again setting its sights on CBD, with 13 Warning Letters going out in 2022. I would expect that number to increase because the year is not yet complete.
Warning Letters are a fairly minor enforcement action. However, the FDA also has the authority to levy fines and even refer cases to the Department of Justice. A Warning Letter can also lead to more severe actions from the FDA, as well as harm a company’s reputation and spook investors.
RELATED: How Would The Cannabis Administration And Opportunity Act Work?
Because of this uptick in Warning Letters, it’s a good time to reevaluate how to mitigate risks of selling CBD. This requires avoiding any direct or indirect claim that CBD has any health or wellness benefits. Direct claims are along the lines of “CBD cures insomnia” or “CBD can treat anxiety.” Indirect claims can be referencing a study regarding CBD, posting customer reviews indicating that CBD has some medical benefit, or implying in any way that CBD does in fact have some positive health or wellness impact.
If you are unsure how to mitigate the risks associated with CBD products, feel free to reach out to our regulatory attorneys for more information.
Daniel Shortt is a corporate and regulatory attorney based in Seattle, Washington who works extensively with entrepreneurs in the cannabis industry. You can contact him at info@gl-lg.com or (206) 430-1336. This article originally appeared on Green Light Law Group and has been reposted with permission.


