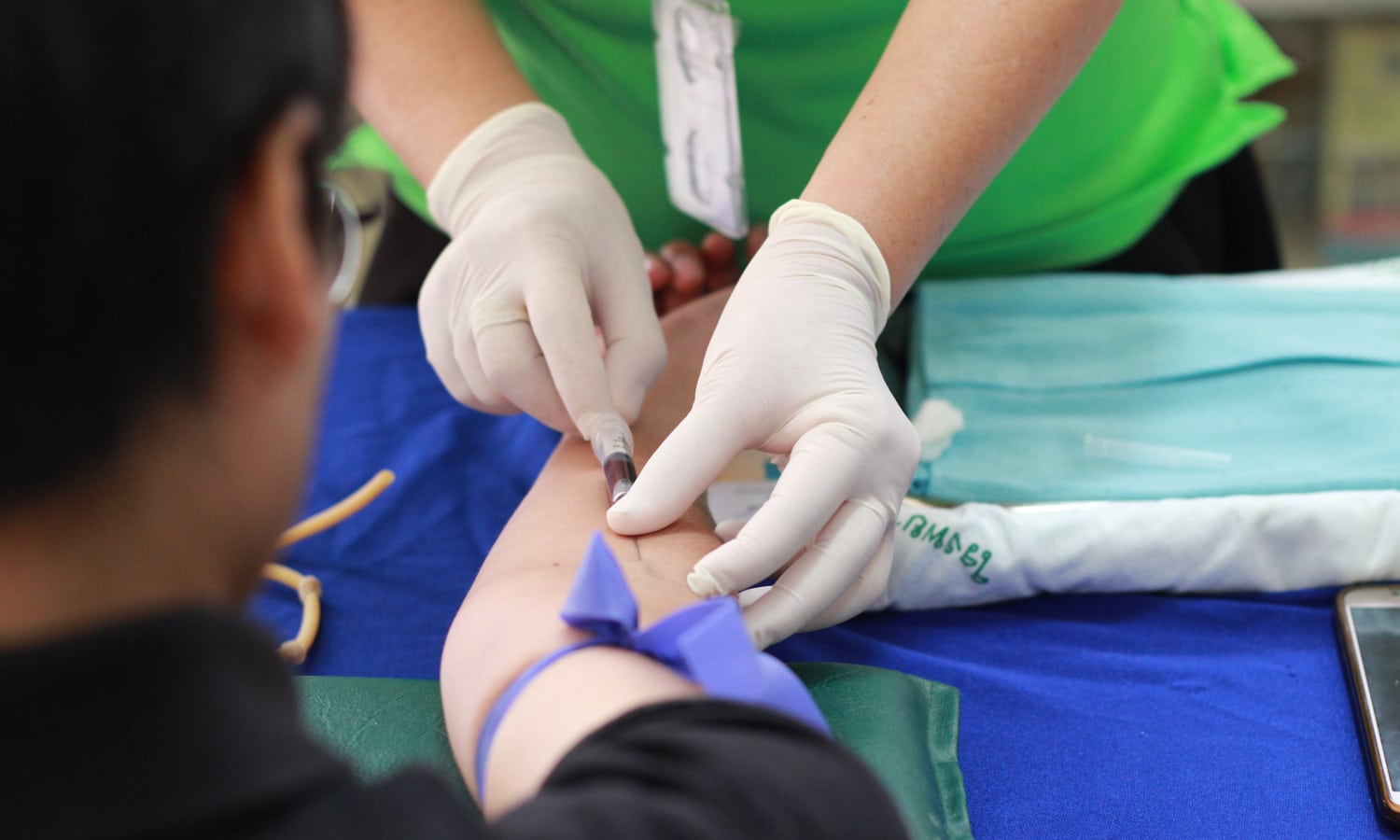
The FDA believes the risk is minimal for donation if donors are in good overall health and practitioners follow screening guidelines already in place.
With over 1.5 million confirmed cases of coronavirus in the United States, the U.S. Food and Drug Administration (FDA) updated its recommendations around specimen collection, as well as convalescent plasma in a recent mid-May update.
Understood to not be caused by blood transfusion, COVID-19 is spread through droplets both in the air or on surfaces. With, “no reported cases of transfusion-transmitted coronavirus” worldwide the FDA’s newest guidance focused on both offering insight into the safety measures in place with blood donation and the importance of encouraging donation, saying, “as communities are affected, it is imperative that healthy individuals continue to donate blood.”
RELATED: Convalescent Plasma: What Is It And Can It Fight COVID-19?
Here are five, key points from the FDA’s recent directive:
- With the lack of SARS-CoV-2 (the name of the coronavirus responsible for causing COVID-19) cases spreading by blood, the FDA believes the risk is minimal for donation if donors are in good overall health and practitioners follow screening guidelines already in place. It was also recommended that, “The blood establishment’s responsible physician must evaluate the prospective donor and determine eligibility.” The FDA illustrated that individuals overcoming COVID-19, or with suspected cases of the disease wait, “blood for at least 14 days after complete resolution of symptoms.” The FDA also shared that, “individuals who are tested and found positive for SARS-CoV-2 antibodies, but who did not have prior diagnostic testing and never developed symptoms, can donate without a waiting period and without performing a diagnostic test (e.g., nasopharyngeal swab).”
- Since donors are already checked for temperature and health, the FDA does not believe donors should be tested to see if they are “asymptomatic.” Revised in early May, the FDA’s policy on antibody tests focuses on prioritization.
- The FDA took a soft stance on clinics offering pamphlets or information, stating, “Blood establishments may wish to consider donor educational materials to instruct individuals to self-defer and refrain from blood donation” if they have come into contact with or are suspected to have COVID-19.
- Recommending clinics consider instructing donors pause from giving blood, “at least 14 days after complete resolution of symptoms or the date of the positive diagnostic test, whichever period is longer,” the FDA is asking blood facilities to be mindful of the spread.
- The FDA summarized they supported the “recommendations of AABB’s Interorganizational Task Force encouraging healthy individuals to make plans to donate blood to maintain the adequacy of the nation’s blood supply.” For individuals that wish to donate, the FDA recommends:
-
- AABB: www.aabb.org
- America’s Blood Centers: www.americasblood.org
- American Red Cross: www.redcrossblood.org
- Armed Services Blood Program: www.militaryblood.dod.mil